|
Lettuce Aphid Spreading
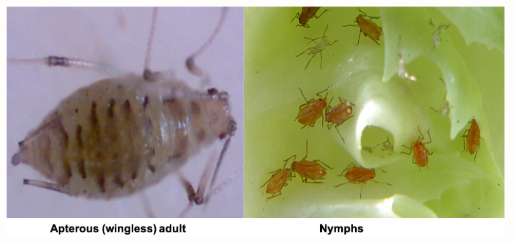
Lettuce aphid (commonly referred to as red aphid) showed up early this year in organic lettuce and had not been reported in conventional lettuce until the past few weeks. It now appears to be spreading and showing up more regularly in lettuce fields throughout the area. Over the past two weeks I have had several conversations with PCAs concerning lettuce aphid in both organic and conventional lettuce with reports of infested fields in Roll, Bard, Dome Valley, Wellton and Yuma Valley. They are beginning to build up to high numbers at the Yuma Ag Center, and in fact are the predominant aphid species present in my lettuce efficacy trials. This is not real common. The last time lettuce aphids were this abundant on desert produce was the spring of 2012. Keep in mind, daytime high temperatures in the 70-80's are ideal for lettuce aphid population growth, and the 14-day weather forecast suggests that lettuce aphid will be around for a while.
A few things to consider about lettuce aphids relative to the other aphids we commonly see. Lettuce aphids prefer to colonize the terminal growth, and generally found in the heads or hearts. Sampling should be focused in the terminal growth of young plants, and in the heads and hearts of older plants. When they are present under ideal temperatures (like right now) they can reproduce prolifically, producing many more winged adults than other aphid species. This is likely why we are beginning increased dispersal and new infestations.
Finally, a few suggestions for management. If your lettuce is young and not closed in yet, consider using an aphicide with contact activity like Sequoia. Assuming sprays can reach new growth, you should see dead aphids with 3-5 days. Also, avoid using pyrethroids on smaller lettuce. This will conserve some of the natural enemies like syrphid fly and lady beetle larvae that may provide some additional control. On older lettuce you best choice is Movento at 5 oz/ac. It is advised that you increase your rate of penetrating adjuvant to 0.375 % vol/vol or higher on larger plants. This should help move the product into the vascular tissue and improve systemic activity. Don’t expect to initially see a lot of dead aphids. It normally requires 7-10 days of activity before significant reduction in the infestation is observed. Sooner when temperatures are in the 80’s. Look for the wingless adults (larger brown forms); their absence is a good sign that you are cycling them out. Obviously, use of pyrethroids is more important on lettuce approaching harvest in order to prevent contamination by trash bugs. For more information on lettuce aphid please refer to Lettuce Aphid on Spring Produce.
 Name that Pest
Name that Pest

Remember, When in Doubt . . . . . “SCOUT”

|
|
Summer Annual Grass Identification
It is better to be a month early when applying preemergence herbicides for summer annual grass control than it is to be a day late. They have begun to emerge so don’t wait. It is important to know which grass(s) that you have but that is not possible without prior knowledge when making preemergence applications. There are more than 25 annual grass species that are found here during the summer although only about 10 are common. Most of these look very similar at early growth stages and identification can be difficult. There is a tendency to lump them all together and call them water grass, jungle rice barnyard grass or several other names. There are differences between them, however, and it is important to accurately identify them at early growth stages. This is important because many of them respond differently to herbicides and have different growth habits. For example, sprangletop is only controlled by the highest rates of Select (clethodim) and generics of this herbicide and missed by all of the other selective postemergence herbicides like Poast and Fusilade and sandbur is tolerant to all of them. By the same token, sandbur and sprangletop can over winter, come back from crowns and not be controlled by preemergence herbicides applied in the spring while most other summer annual grasses die in the winter and the seed can be controlled with herbicides in the spring. There are just 6 genera and 12 species of summer annual grass that are common here. These include echinochloa (water grass and barnyard grass), leptochloa (red sprangletop and mexican sprangletop), eriochloa (southwestern cupgrass and prairie cupgrass), cenchrus (field sandbur and red sandbur), setaria (green foxtail and yellow foxtail), and chloris (feather finger grass and truncate finger grass). A power point presentation can be found by clicking on Summer Annual Grass ID which contains pictures and descriptive characteristics of each of these species.
|
|
Botrytis Crown Rot of Lettuce

Botrytis rot is not considered a major problem in lettuce, but it can cause significant damage/loss when the field conditions are favorable for the pathogen. Cool wet conditions are favorable for the pathogen. Symptoms include water-soaked, brownish-gray to brownish-orange, soft wet rot that occurs on the oldest leaves in contact with the soil. Old leaves are more susceptible than young leaves and the fungus can move into the healthy parts. Fuzzy gray growth can be observed in the disease area which is characteristic of the pathogen. In worse cases, the entire plant can collapse. Romaine cultivars, transplanted lettuce that are big and have leaves touching the soil are more susceptible.
The pathogen: Botrytis cinerea
Botrytis cinerea affects most vegetable and fruit crops, as well as a large number of shrubs, trees, flowers, and weeds. Outdoors Botrytis overwinters in the soil as mycelium on plant debris, and as black, hard, flat or irregular sclerotia in the soil and plant debris or mixed with seed. The fungus is spread by anything that moves soil or plant debris, or transports sclerotia. The fungus requires free moisture (wet surfaces) for germination, and cool 60 to 77 F, damp weather with little wind for optimal infection, growth, sporulation, and spore release. Botrytis is also active at low temperatures and can cause problems on vegetables stored for weeks or months at temperatures ranging from 32 to 50. Infection rarely occurs at temperatures above 77 F. Once infection occurs, the fungus grows over a range of 32 to 96 F.
Masses of microscopic conidia (asexual spores) are produced on the surface of colonized tissues in tiny grape-like clusters (see picture) They are carried by humid air currents, splashing water, tools, and clothing, to healthy plants where they initiate new infections. Conidia usually do not penetrate living tissue directly, but rather infect through wounds, or by first colonizing dead tissues (old flower petals, dying foliage, etc.) then growing into the living parts of the plant.
Disease management:
1. Buy high-quality seed of recommended varieties. Treat the seed before planting.
2. Practice clean cultivation. Plant in a light, well-drained, well-prepared, fertile seedbed at the time recommended for your area. If feasible, sterilize the seedbed soil before planting, preferably with heat. Steam all soil used for plant beds at 180 F (81 C) for 30 minutes or 160 F (71 C) for one hour.
3. Avoid heavy soils, heavy seeding, overcrowding, poor air circulation, planting too deep, over-fertilizing (especially with nitrogen), and wet mulches.
4. Focus on healthy plant vigor. Do not over fertilize.
5. Use drop or furrow irrigation instead of sprinklers. If sprinklers have to be used, irrigate morning or early afternoon giving enough time for foliage to dry.
6. Apply recommended fungicides when conditions favor disease development. Make sure to rotate fungicide to avoid development of resistance.
|
|
Real IPM
 Do you like the IPM Updates? Please let us know, VOTE
HERE
Do you like the IPM Updates? Please let us know, VOTE
HERE
Send your questions to:
CALS-Yuma-AZVegIPM@email.arizona.edu
|
|
|
The Vegetable IPM Updates Archive page provides links
to updates from previous weeks.
The Vegetable IPM Video Archive page contains a collection
of educational videos from current research work in vegetable crops by University
of Arizona Researchers.
|
For questions or comments on any of the topics please contact Marco Pena at the Yuma Agricultural Center.
College of Agriculture, The University of Arizona, Tucson, AZ.
|
|
|

|
Home
Cotton
| Veggies |
Forages
| Grains
| Citrus |
Crop x Crop
Insects |
Diseases
| Weeds |
Pesticides
| Economics |
News
| Weather |
Research
| Photos
| Contacts |
General Info.
document located at: http://cals.arizona.edu/crops/vegatables/advisories/archive.html
Copyright © 2001 University
of Arizona,
College of Agriculture and Life
Sciences
Webmaster: Al Fournier (acis@ag.arizona.edu)
|
|