
 |
| |
| Activity G-2: | Contamination of an Aquifer |
| This youth activity is one in a series of two activities that can be used to introduce groundwater concepts. It is a good introductory activity and can be adapted for all grades. | |
| Purpose: | |
| To illustrate how water flows through an aquifer, how groundwater can become contaminated, and how difficult it is to clean up contamination. | |
| Background: | |
| Many communities obtain their drinking water from underground sources called aquifers. Water suppliers drill wells through soil and rock into aquifers for the groundwater contained in the aquifer. Unfortunately, the groundwater can become contaminated by harmful chemicals that percolate down through soil and rock into the aquifer - and eventually into the well. Groundwater contamination by chemicals is caused by industrial, agricultural, and urban runoff and/or the improper management of chemicals, including improper disposal of household chemicals, such as lawn care products and cleaners. Such contamination can pose a significant threat to human health. The measures that must be taken by utilities to either protect or clean up contaminated aquifers are quite costly. | |
| Materials: | |
| Procedure: | |
|
|
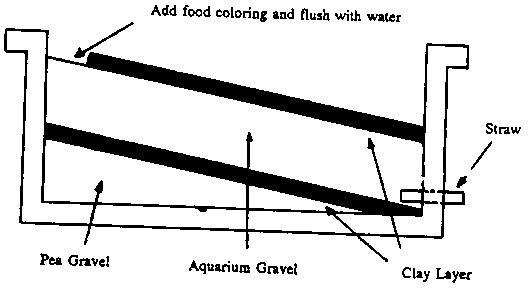 |
|
| Discussion: | |
| Before the Activity | |
|
|
| After the Activity | |
|
|
| Extensions: |
|
| This activity was adapted by Dr. Kitt Farrell-Poe from Science Demonstration Projects in Drinking Water (Grades K-12) by the US Environmental Protection Agency, Office of Water, EPA 570/9-90-007, April 1990. |
|
Issued in furtherance of Cooperative Extension work, Acts of May 8 and June 30, 1914, in cooperation with the U.S. Department of Agriculture, James A. Christenson, Director, Cooperative Extension, College of Agriculture, The University of Arizona. The University of Arizona College of Agriculture is an Equal Opportunity employer, authorized to provide research, educational information, and other services only to individuals and institutions that function without regard to sex, race, religion, color, national origin, age, Viet Nam Era Veteran's status, or disability.
|