 The Nitrogen Cycle - October 5, 2016 Jeff Schalau, Agent, Agriculture & Natural Resources University of Arizona Cooperative Extension, Yavapai County All living cells need nitrogen to make nucleic acids, proteins, and other cellular constituents. Plants absorb nitrogen and put them into amino acids and proteins. Other organisms consume the plants. In their stomachs, the nitrogen containing proteins are broken down into amino acids again and recycled to make new proteins and nucleic acids. When the plants shed leaves and animals produce waste and when living organisms die, decomposers (fungi, bacteria, protozoa, insects, worms, etc.) break down these materials into plant available nitrogen (nitrate and ammonium) which can be reabsorbed by plants. Although the total amount of nitrogen on Earth is fixed, there is an abundant supply in the earth's atmosphere - nearly 79% in the form of nitrogen gas (N2). However, N2 is unavailable for use by most organisms because the two nitrogen atoms are held together by very strong and stable chemical bonds. This makes the N2 molecule effectively inert. In order for nitrogen to be used for plant growth it must be "fixed" in the form of ammonium (NH4) or nitrate (NO3) ions. Microorganisms play an important role in converting the inert atmospheric nitrogen into plant available nitrogen. This process is called nitrogen fixation. Biological nitrogen fixation occurs primarily in agricultural fields (35%), forested lands and wildlands (20%), and the oceans (14%). Non-living factors also convert N2 into plant available nitrogen through lightning (4%), combustion (8%), and industrial fertilizer production (20%). The biological nitrogen fixation process is driven primarily by bacteria which are either free-living or symbiotically associated with plants. The most familiar of these symbiotic associations is between Rhizobium bacteria and plants in the legume family. Some examples of legumes are beans, peas, mesquite trees, catclaw, clover, and alfalfa. Whatever pathway is taken, the nitrogen must be converted from N2 to a plant available form before it can be used by plants. After plant available forms of nitrogen, ammonium and nitrate, are in the soil, there are three things that can happen: (1) plants may take it up, (2) it may be leached below the root zone by water, or (3) denitrifying bacteria can use it for an energy source and release it back to the atmosphere as N2. The nitrogen released to the atmosphere may start the cycle over again. Any nitrogen leached below the root zone and could become a contaminant in groundwater. Gardeners use the nitrogen cycle to their advantage when they use compost or plant cover crops. In a compost pile, the same microorganisms (bacteria, fungi, and protozoa) and invertebrates (worms and insects) present in the adjacent soil break down the organic matter into proteins and amino acids. Ultimately, the microorganisms break it down into nitrate and ammonium which can be taken up again by plants. Whether it is a dead organism or manure, soil microorganisms regulate the release of plant available nitrogen from decomposing. Decomposition during the nitrogen cycle is a timed-release process. Decomposition of organic matter by microorganisms is regulated by soil temperature – the warmer the soil, the faster the decomposition. Synthetic nitrogen fertilizers are sold through nurseries and garden centers. These fertilizers are made through industrial processes and are available at lower soil temperatures. Synthetic nitrogen fertilizers are often blended, coated, or otherwise processed to make the various products available at nurseries and garden centers. Remember, nitrogen fertilizer encourages leaf and shoot growth. Most trees and shrubs do not require nitrogen to grow normally. Too much nitrogen can result in excessive growth, weaker wood, plants that require more frequent pruning, and decreased flower and fruit production. Our vegetable and flower gardens are artificial environments that require higher amounts of nutrients – including nitrogen. Understanding how nitrogen is cycled through plants, animals, and soil will help you effectively apply fertilizers. Go to the on-line version of this column for a graphic representation of the nitrogen cycle. Follow the Backyard Gardener on Twitter – use the link on the BYG website. If you have other gardening questions, call the Master Gardener help line in the Camp Verde office at 928-554-8992 or e-mail us at verdevalleymg@gmail.com and be sure to include your name, address and phone number. Find past Backyard Gardener columns or provide feedback at the Backyard Gardener web site: http://cals.arizona.edu/yavapai/anr/hort/byg/. 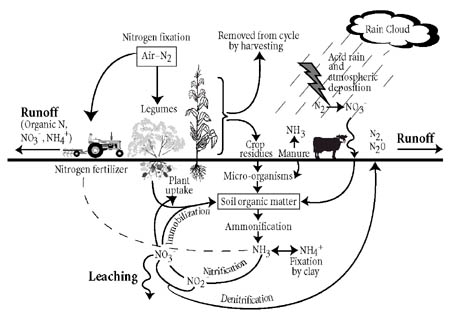 |