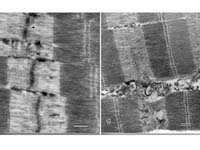
| Characterizing Caplains Implications for production agriculture and human health Calpains and calpastatin may not be household words but their action is as close to you as your own muscles. Calpains are calcium-dependent enzymes that are capable of destroying certain proteins in cells. Calpastatin is an inhibitor or brake that slows or hinders the ability of the calpains to destroy cellular proteins. In animals, these calcium-dependent enzymes and their inhibitor affect the way the muscle tissue grows or wastes away. If calpain activity is low or closely regulated, healthy muscle growth is the result, but unregulated calpain activity culminates in loss of muscle tissue and contributes to the development of certain diseases. "In humans and animals, skeletal muscles are constantly being made and degraded," says Darrel Goll, a professor in the Department of Nutritional Sciences at The University of Arizona who has studied calpains for 20 years. "Muscle protein is part of this process. In humans, up to five to six percent of muscle mass per day is chewed up just to be replaced. This amount of turnover is rather remarkable. If you make more muscle tissue than the five to six percent, you grow. If you make less than that, you waste. Growth is the balance." Since the 1970s, when calpains and calpastatin were first identified, scientists around the world have worked to describe their action more fully and perhaps develop pharmaceutical ways of regulating them. This basic research, carried out at the UA and other institutions may lead to advances in treating heart disease, strokes, muscular dystrophy, and also contribute to improvements in the way domestic animals develop muscles consumed as meat. In the laboratory, Goll and his associates, Parker Antin, Constance Temm, Valery Thompson, Mei Cong, and Jinyang Cong use a variety of tissues including human placental tissue, bovine skeletal muscle, and blood platelets from humans or cows. They watch how the calpains and calpastatin are produced, how they behave, and how genes may be disrupted. They notice what happens when the balance of calpain activity to calpastatin activity changes."We're studying how they work and how their activity is controlled in
living cells." he says. "We're attempting to identify and characterize
their action, and we're also looking at the way calpains and calpastatin
behave in promoting or reducing muscle growth. It seems that anything
in the cell that Research in the past seven years has identified eleven molecules that seem to be related to the calpain family. None of these newly identified molecules has been characterized scientifically. One of them is found only in skeletal muscle and has been identified as the gene involved in a particular type of muscular dystrophy called limb girdle muscular dystrophy, Type 2A. Although the gene has been identified, the protein has not been obtained despite efforts by Goll and his laboratory and a number of laboratories around the world. Goll says there's enough calpain in muscle tissue to destroy it in ten minutes, yet this doesn't happen, so it seems that only one or two percent of the calpain in muscle is active at any one time and that something is keeping the rest of it turned off. Scientists believe that's what calpastatin does. When it's absent or its action is repressed, calpains in muscles go wild. In growth, it's not how fast muscle tissue is formed, but the rate of breakdown that frequently determines whether animals grow and how fast they grow, according to Goll. In animal agriculture, the calpain system also affects the quality of the meat. Although animals can grow faster without degrading proteins in their systems, such circumstances may also result in tougher meat. Goll believes it will be possible to develop products to enhance the rate and efficiency with which domestic animals deposit edible skeletal muscle. However, he believes there's a long way to go in completely understanding the way calpains and calpastatin actually work. "The only thing we know for sure that calpains do is make meat tender," Goll admits. "The interest in their activity lies more in learning what they do and how they do it." Goll's research is sponsored by the USDA for agricultural applications in domestic animal growth and by the Muscular Dystrophy Association and Bayer for human health purposes. The UA research may lead to advances and new products in both fields. "The interest in the health field is enormous right now," Goll says. "It's 90 percent sure that much of the muscle-wasting activity in Duchenne Muscular dystrophy and probably in other muscular dystrophies such as Lou Gehrig's disease is from unregulated calpain activity." He adds that heart attacks and strokes also result in muscle damage that is caused from runaway calpain activity, and that although calpain-inhibiting drugs would not repair the tissues, they would reduce tissue damage and reduce recovery time. "If such drugs could be administered soon enough after vessel blockage and loss of blood flow, there would be much less damage to the heart," Goll explains. "Although such drugs would not prevent heart attacks, they could reduce tissue damage and improve recovery." The research continues, and every discovery sheds more light on the way calpains work and how they might be regulated. In studies just completed, Goll and his associates have found that in embryonic skeletal muscle, calpains are necessary to form muscle cell fusion. It enables the cell in its early stages to continue fusing and to eventually to form a mature muscle cell. Because they are essential for muscle development in embryos, the calpains are not always the "bad guys" nor is calpastatin the "good guy." Together, they form a part of a complex balance of proteins that is essential in stimulating and sustaining muscle development. Article written by Susan McGinley, ECAT, College of
Agriculture Darrel Goll, Department of Nutritional Sciences |
 disturbs the regulation
of calcium seems to result in unregulated calpain activity. That's the
current theory."
disturbs the regulation
of calcium seems to result in unregulated calpain activity. That's the
current theory."