|
| |
| Activity D-2: | How Drinking Water is Cleaned |
| This youth activity is one in a series of two activities that illustrate drinking water concepts. It is a good introductory activity and can be adapted for all grades. | |
| Purpose: | |
| To demonstrate the procedures that municipal water plants use to purify water for drinking. | |
| Background: | |
| Water in lakes, rivers, and swamps often contain impurities
that make it look and smell bad. The water may also contain bacteria
and other microbiological organisms that can cause disease. Consequently,
water from surface sources must be "cleaned" before it can be consumed
by people. Water treatment plants typically clean water by taking
it through the following processes: 1) aeration; 2) coagulation; 3) sedimentation;
4) filtration; and 5) disinfection.
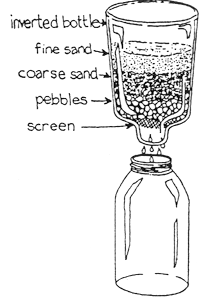
Aeration is the addition of air to water. It allows gases trapped in the water to escape and adds oxygen to the water. Coagulation is the process by which dirt and other suspended solid particles are chemically "stuck together" into so that they can be removed from water. Sedimentation is the process that occurs when gravity pulls the particles of floc (clumps of alum and sediment) to the bottom of the cup. Filtration through a sand and pebble filter removes most of the impurities remaining in water after coagulation and sedimentation have taken place. In most municipal water treatment plants, disinfection is the last step before distributing the water. This activity demonstrates the first four water treatment processes. |
|
| Materials: | |
| Procedure: | |
|
|
| Extension: | |
| This activity was adapted by Dr. Kitt Farrell-Poe from Science Demonstration Projects in Drinking Water (Grades K-12). EPA 570/9-90-007. | |
|
Issued in furtherance of Cooperative Extension work, Acts of May 8 and June 30, 1914, in cooperation with the U.S. Department of Agriculture, James A. Christenson, Director, Cooperative Extension, College of Agriculture, The University of Arizona. The University of Arizona College of Agriculture is an Equal Opportunity employer, authorized to provide research, educational information, and other services only to individuals and institutions that function without regard to sex, race, religion, color, national origin, age, Viet Nam Era Veteran's status, or disability.
|
|