 Water and Plants - November 8, 2017 Jeff Schalau, Agent, Agriculture & Natural Resources University of Arizona Cooperative Extension, Yavapai County I spend a lot of time talking and writing about the need to properly irrigate landscapes, gardens, and orchards. However, I have spent little time telling folks why water is so important to plants and life in general. About 90% of the water taken up by plants simply passes through and is released back to the atmosphere. This water allows the plant to bring in a continuous supply of mineral nutrients from the soil. It also keeps leaf openings (stomata) open to allow carbon dioxide to enter the plant for photosynthesis. The other 10% of the water is used to conduct chemical reactions that are necessary for growth and maintenance. Water is a fantastic biological solvent sometimes called the “universal solvent” because it is capable of dissolving more substances than any other liquid on Earth. The water molecule consists of two hydrogen atoms bonded to a single oxygen atom. The hydrogen atoms are not on opposite sides of the oxygen, but are separated by an angle of 105 degrees. This causes the oxygen atom to have a slight negative charge and the two hydrogen atoms to have a slight positive charge. This slight polarity of the water molecule is what causes water molecules to be attracted to each other and to other objects having a slight charge. Big deal, you say? It is a big deal because this polarity allows water to become an ideal biological solvent: most chemical reactions in living organisms need water as a solvent. Products of these biochemical reactions include: (1) fats and oils (lipids), (2) proteins (enzymes and structural materials), (3) sugars and cellulose (carbohydrates), and (4) nucleic acids (DNA and RNA). These are the building blocks for all living organisms. Proteins, sugars, and nucleic acids are water soluble. Lipids are largely non-polar and repel water in special ways. This makes them ideal for cell membranes which compartmentalize one area of a living cell from another. Lipids are also excellent in their capacity to store energy. Of the 17 (or 18, depending on plant species and research findings) known essential plant nutrients, 13 regularly enter the plant through the soil. These essential nutrients are in ionized forms which are polar making them readily soluble in water. In soil, water forms a solution with these mineral nutrient ions. Under normal soil/water conditions, the concentration of dissolved materials is greater inside the plant than in the soil. This causes the water/nutrient solution to cross the root membranes and into the plant due to osmosis. Water/nutrient uptake is usually impeded under saline soil conditions. Once inside the plant, the attraction of water molecules for each other drives transpiration. As water molecules are evaporated from the leaf surface, negative pressure is created and this pulls other water molecules higher up the plant through the xylem. Mineral nutrients are passively carried along with the water. Plant cells remove the mineral nutrients from solution once they reach a location where they are needed. Water contributes electrons that combine with carbon dioxide to create carbohydrates during photosynthesis. These carbohydrates form the building blocks for proteins, lipids, and nucleic acids. Carbohydrates are also used for energy needed to drive other chemical reactions and processes within the plant. Plant sap (not pitch) is water-based and rich in sugars and proteins. These compounds are moved to other locations within the plant via the phloem. In a woody plant (trees and shrubs), xylem is the wood and phloem is in the inner bark. Non-woody plants have a specialized plumbing system that contains xylem and phloem in tissues called vascular bundles. The phloem redistributes the sugars and proteins to other areas of the plant where they can be used or stored. Thinking about water in this way should create a greater appreciation for its importance. It is the universal solvent on the planet earth. I have included additional information with the online edition (see URL below). Follow the Backyard Gardener on Twitter – use the link on the BYG website. If you have other gardening questions, call the Master Gardener help line in the Camp Verde office at 928-554-8992 or e-mail us at verdevalleymg@gmail.com and be sure to include your name, address and phone number. Find past Backyard Gardener columns or provide feedback at the Backyard Gardener web site: http://cals.arizona.edu/yavapai/anr/hort/byg/. Graphics 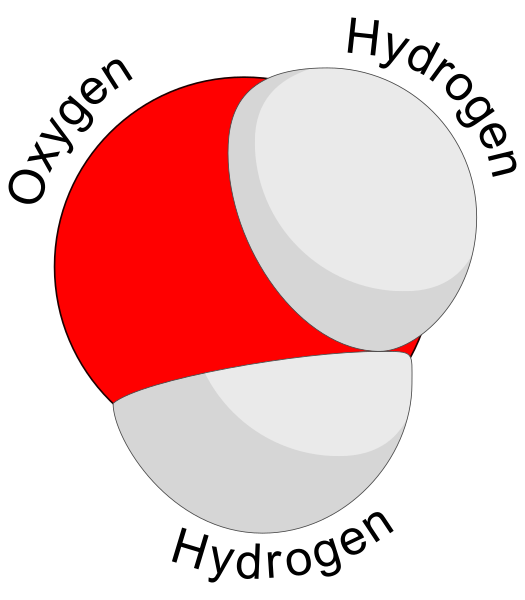 Three dimensional model of a water molecule (Image from Creative Commons: https://commons.wikimedia.org/wiki/File%3AWater_molecule_(1).svg).
Three dimensional model of a water molecule (Image from Creative Commons: https://commons.wikimedia.org/wiki/File%3AWater_molecule_(1).svg).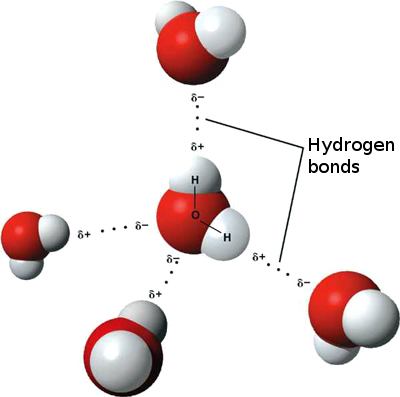 Water molecules showing weak attractions between adjacent water molecules called "hydrogen bonding". (Image from Creative Commons: https://upload.wikimedia.org/wikipedia/commons/f/f9/3D_model_hydrogen_bonds_in_water.jpg).
Water molecules showing weak attractions between adjacent water molecules called "hydrogen bonding". (Image from Creative Commons: https://upload.wikimedia.org/wikipedia/commons/f/f9/3D_model_hydrogen_bonds_in_water.jpg).Additional Resources Soil, Water, and Plant Relationships K-State Research and Extension www.ksre.k-state.edu/irrigate/reports/L904.pdf Properties of Water Utah State University Extension extension.usu.edu/waterquality/learnaboutsurfacewater/propertiesofwater/ |